Alzheimer’s Research Sheds New Light on Tau Protein Turning into Disease State
Image Courtesy: Science.org
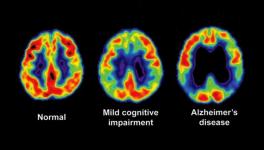
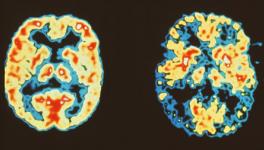
Alzheimer’s disease (AD), the most common form of dementia, affects a big chunk of the global population. Despite being widespread, the disease has no effective therapy as such due to gaps in our understanding of the molecular mechanism of its progression in the brain.
Nevertheless, decades of research have revealed certain traits of the neurodegenerative disease, especially about what happens in the brain at its onset, and one of the hallmarks is the Tau protein’s deformation.
Now, researchers have added a new aspect to Tau turning from a normal state to a diseased state. Researchers believe that the more information we have on the protein, the better it is to understand the disease and discover potential therapies.
Researchers also believe that stopping the Tau from proceeding into the diseased state may prove to be a potential therapy for AD in the future. The findings were published recently in Science Advances and the research was carried out by scholars of Finders University, Australia.
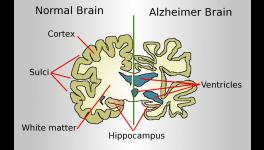
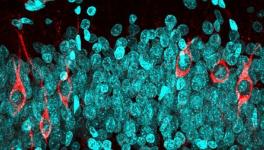
AD progression leads to an accumulation of Tau protein inside brain deposits with the protein heavily modified and causing memory disruptions. The Tau anomaly has been known to researchers but how the protein reaches the deformed states has remained elusive.
“Alongside a small peptide called amyloid-beta, Tau protein is a central factor in Alzheimer’s disease. Tau is necessary for the toxic effects on brain cells that result in impaired memory function,” said Dr Arne Ittner, a senior research fellow in neuroscience at the Flinders Health and Medical Research Institute and one of the authors of the study.
The study attempted to answer whether a change at a specific location of Tau would lead to subsequent changes of it in other positions. The researchers tried to decipher the relationship between Tau and protein kinases, which are special enzymes that introduce changes in Tau.
“Usually, protein kinases target specific spots called phosphorylation sites in Tau and other proteins and introduce changes only at these specific spots. However, we suspected that some of these enzymes are able to target several spots in Tau and would do so even more efficiently if Tau were already modified at one spot to begin with,” said the study’s lead author Dr Kristie Stefanoska.
The researchers carried out a large experiment, including 20 different changes in Tau and 12 enzymes, and found the most abundant changes in Tau in the brains of AD patients. The researchers found that there are certain changes in the Tau which helped in further changes of the protein. Along with this, the researchers also found certain master sites in Tau which can induce subsequent changes at most of the other sites of the protein.
“By modifying these master sites, we were able to drive modification at multiple other spots within Tau, leading to a similar state seen in the brains of Alzheimer’s patients,” said Ittner explaining the importance of such positions in Tau.
Experts believe that this is a new concept in Tau deformation in AD. However, to explore its therapeutic potential, further detailed investigations are necessary. “Tau modification in Alzheimer’s disease is a complicated process. Ours is the first study to link an initial change in Tau with multisite modification along the entire protein,” said one of the authors of the study.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.